The University of Namur's electron microscopy platform is open to all researchers wishing to use these techniques to observe or analyze samples for research or teaching purposes. It also makes its equipment and expertise available to other academic institutions, companies, and industries for the analysis of their samples.
For more than 50 years, electron microscopy has been at the heart of research at UNamur. This technology uses a beam of electrons instead of light to illuminate the sample. Electrons have a much shorter wavelength than visible light, which allows for nanometric or even atomic resolutions to be achieved.

Beyond imaging, electron microscopy has been enhanced with complementary analytical techniques that provide chemical, structural, and spectroscopic information about the samples being studied. These include energy dispersive X-ray spectroscopy (EDS), which provides additional information on the elemental composition of the sample, and electron backscatter diffraction (EBSD), a crystallographic method used to determine grain orientation, crystal structure, and the phases present in a material.
Today, the platform is equipped with three transmission electron microscopes and two scanning electron microscopes with different analytical probes, as well as the necessary equipment for sample preparation. These tools make electron microscopy a versatile instrument, indispensable in fields as varied as materials science, cell biology, nanotechnology, and geology. They not only allow structures to be visualized, but also provide insight into their composition, organization, and functional properties.
Facilities
The electron microscopy platform team
The electron microscopy platform team is made up of experts in the various technologies used. It includes an engineer who supervises the analyses carried out on the microscopes and a technician responsible for preparing the samples.
Reservations
The conditions of access and use of microscopes and other equipment in the department by members of the University are governed by regulations. Anyone wishing to carry out analyses in the department is invited to familiarize themselves with these regulations.
For internal members of the University of Namur, procedures, information, and user guides are available on the intranet (restricted access).
For external users (analysis requests), please contact the service manager:
Jean-François Colomer - jean-francois.colomer@unamur.be - Tel: +32 (0)81 72 47 08
Spotlight
News

Producing "green" hydrogen from water from the Meuse River? It's now possible!
Producing "green" hydrogen from water from the Meuse River? It's now possible!
At UNamur, research is not confined to laboratories. From physics to political science, robotics, biodiversity, law, AI, and health, researchers collaborate daily with numerous stakeholders in society. The goal? Transform ideas into concrete solutions to address current challenges.

Focus #2 | What if our rivers became a source of clean energy for the future?
An international team of chemistry researchers, led by Dr. Laroussi Chaabane and Prof. Bao-Lian Su, has just demonstrated that it is possible to produce "green" hydrogen using natural water and sunlight. These findings have been published in the prestigious Chemical Engineering Journal.
When sunlight becomes a source of clean energy
Faced with climate change, pollution, and energy shortages, the search for alternatives to fossil fuels has become a global priority in order to achieve carbon neutrality by 2050. Among the solutions being considered, green hydrogen appears to be a particularly promising energy carrier: it has a high energy density and can be produced without greenhouse gas emissions. Today, most of the world's hydrogen (around 87 million tons produced in 2020) is obtained through costly and polluting electrochemical processes, mainly used by the chemical industry or fuel cells. Hence the major interest in more sustainable methods.
Water photocatalysis: the "Holy Grail" of chemistry
Producing hydrogen and oxygen directly from water using light, a process known as photocatalysis of water, is often referred to as the "Holy Grail of chemistry" because it is so complex to master. At the University of Namur, researchers at the Laboratory of Inorganic Materials Chemistry (CMI), part of the Nanomaterials Chemistry Unit (UCNANO) and the Namur Institute of Structured Matter (NISM), have taken a decisive step forward. They have demonstrated that it is possible to use natural water, and no longer just ultrapure water, to produce green hydrogen under the action of sunlight.

The core of the process is based on an innovative photocatalyst, which acts as a kind of "chemical pair of scissors" capable of splitting water molecules into hydrogen and oxygen—an area in which the CMI laboratory has recognized expertise.
A 3D photocatalyst based on graphene and gold
The new material developed is a three-dimensional (3D) photocatalyst based on titanium oxide, graphene, and gold nanoparticles. This 3D architecture allows for better light absorption and more efficient generation of free electrons, which are essential for triggering the water dissociation reaction. One of the main challenges lies in the use of natural water, which contains minerals, salts, and organic compounds that can disrupt the process. To address this challenge, the researchers tested their device with water from several Belgian rivers: the Meuse, the Sambre, the Scheldt, and the Yser.
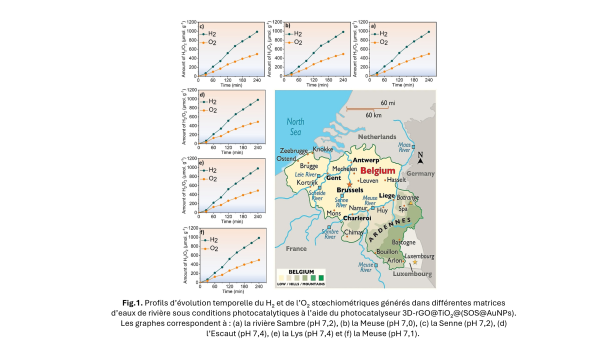
A remarkable result and a first in Belgium!
The performance achieved is almost equivalent to that measured with pure water.
This is a first in Belgium, opening up concrete prospects for the sustainable use of local natural resources!
The full article, "Synergistic four physical phenomena in a 3D photocatalyst for unprecedented overall water splitting," is available in open access.
International recognition
This scientific breakthrough also earned Dr. Laroussi Chaabane the award for best poster at the 4th International Colloids Conference (San Sebastián, Spain, July 2025), highlighting the impact and originality of this work.
An international research team
- University of Namur, Faculty of Sciences, UCNANO, Laboratory of Inorganic Materials Chemistry (CMI) and Namur Institute of Structured Matter (NISM), Belgium | Principal Investigator (PI) | Professor Bao Lian SU; Postdoctoral Researcher | Dr. Laroussi Chaabane
- Institute of Organic Chemistry, Phytochemistry Center, Academy of Sciences, Bulgaria
- Department of Organic Chemistry (MSc), Loyola Academy, India
- Free University of Brussels (ULB) and Flanders Make, Department of Applied Physics and Photonics, Brussels Photonics, Belgium
- University of Quebec in Montreal (UQAM), Department of Chemistry, Montreal, Quebec, Canada
- National Institute for Scientific Research - Energy Materials Telecommunications Center (INRS-EMT), Varennes, Quebec, Canada
- Wuhan University of Technology, National Laboratory for Advanced Technologies in Materials Synthesis and Processing, China
What next?
At this stage, the study constitutes proof of concept demonstrating the feasibility of the process. It illustrates the excellence of chemical engineering and nanomaterials research at UNamur, as well as its potential for sustainable energy applications. A new study is underway to evaluate the performance of the process with seawater, a key step towards large-scale green hydrogen production.
State-of-the-art equipment
The analyses carried out were made possible thanks to the equipment available at UNamur's Physico-Chemical Characterization (PC²), Electron Microscopy, and Material Synthesis, Irradiation, and Analysis (SIAM) technology platforms. UNamur's technology platforms house state-of-the-art equipment and are accessible to the scientific community as well as to industries and companies.
The authors would like to thank the Wallonia Public Service (SPW) for its ongoing commitment to scientific research and innovation in Wallonia, enabling UNamur to develop technological solutions with a significant societal and environmental impact.
From fundamental to applied research, UNamur demonstrates every day that research is a driver of transformation. Thanks to the commitment of its researchers, the support of its partners from all walks of life, funders, industrial partners, and a solid ecosystem of valorization, UNamur actively participates in shaping a society that is open to the world, more innovative, more responsible, and more sustainable.
To go further
This article complements our publication "Research and innovation: major assets for the industrial sector" taken from the Issues section of Omalius magazine #39 (December 2025).

Colourful speleothems: treasures hidden deep within the earth
Colourful speleothems: treasures hidden deep within the earth
Well hidden from passersby, caves nevertheless conceal particularly aesthetic secrets. For the past four years, Martin Vlieghe has been pursuing a PhD in geology at UNamur. He is exploring the origin of the surprisingly varied colours of certain concretions nestled in the heart of Belgian and French caves. Together with Prof. Johan Yans and Gaëtan Rochez, he samples, observes, and analyses these magnificent objects with the aim of uncovering the mysteries they conceal.

Photo: Green speleothems in the Aven du Mont Marcou (Hérault, France) © Stéphane Pire, Gaëtan Rochez (UNamur)
Speleothems, for instance stalactites and stalagmites, are commonly composed of calcite or aragonite (CaCO3). This mineral compound comes directly from the rock in which the cave was formed and naturally has a white to brownish colour. However, speleothems can sometimes exhibit unique and unusual colours. From yellow to black, blue, red, green, and even purple, there is something for everyone!
Such a diversity of colours reflects the many possible causes: mineralogical, chemical, biological, or even physical. A speleothem, like any natural formation, is never perfectly pure. Their deposition process, through the precipitation of calcium carbonate dissolved in water, is necessarily accompanied by the deposition of numerous impurities carried along with the water circulating underground. Even if these impurities are sometimes too low in concentration or simply uncoloured, they can still have a visible impact on the colour.
OK, but what is the point?
The formation of speleothems is very often linked to impurities dissolved in groundwater. Therefore, studying coloured speleothems provides valuable information about potential contamination of surface water with heavy metals or other harmful organic compounds, which in some cases may be consumed by residents. It is therefore a simple and direct way to identify areas with potentially contaminated water and to determine whether this contamination poses an environmental or health risk.
This is the objective of Martin Vlieghe's thesis: to apply a range of cutting-edge analytical techniques to samples of these speleothems to determine these causes and propose an explanation for the origin of the colouring elements.
Here are a few examples.
Green from the Aven du Marcou: the influence of nickel
An initial project explored the green speleothems of the Aven du Marcou (see photo above). Located in the Hérault department of France, this chasm is well known in the area for its series of impressive shafts, the largest of which is over 100 meters deep. It also has a tiny chamber hidden at the top of a steep wall, which houses an impressive concentration of deep green speleothems. After all the effort of descending and climbing ropes to progress through this very vertical cave, what a wonderful reward to discover this true underground gem! Once the initial wonder has passed, it's time to get to work! We observe, describe, interpret, and collect a few green fragments from the ground, while respecting the integrity of the site as much as possible. Back in Belgium, it's time to move on to the analyses.
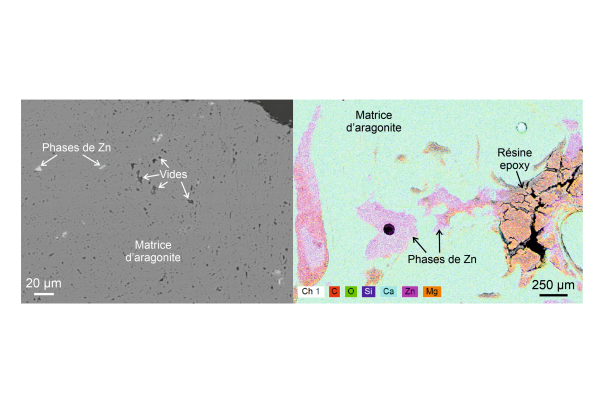
Careful observation of the recovered fragments quickly reveals the presence of green minerals in the outer part of the speleothems, which are easily associated with the green colour observed. These minerals, which are deposited in platelets parallel to the white aragonite (CaCO₃), turn out to be nepouite crystals, a nickel phyllosilicate ((Ni,Mg)₃Si₂O₅(OH)₄) usually found in marine volcanic rocks.

The discovery is all the more surprising given that there are no nickel deposits in the vicinity of the cave! Further study of the composition of the nepouite reveals that they contain a high concentration of zinc, which is also very unusual and suggests that they are in fact quite different from those commonly mined in volcanic deposits. Finally, this mystery was solved by a thorough examination of the rock outcrops in the immediate vicinity of the cave. Just above the cave are siliceous deposits particularly rich in pyrite, an iron sulphide commonly found in this type of settingst. Analysis of these sulphides reveals high concentrations of nickel, which is also found in the natural water source closest to the cave.
The result of this "investigation" and final explanation: nepouite was able to settle underground through the dissolution of various chemical elements contained in the pyrite of the overlying rocks, which were transported into the cave by surface water and were able to crystallize on site.
Malaval blues: when metals interact
The Malaval cave is very different from the Aven du Marcou. Located in Lozère (France), it extends largely along a high underground river that winds beneath the Cévennes massif. At the bend of a meander, one can find magnificent blue speleothems.
As in the Aven du Marcou, the coloured speleothems are found only in two specific locations in the cave and nowhere else, suggesting that the origin of the chromophore elements is probably very localized.

Photos - Left: Blue stalagmite in Malaval Cave. Right: Cluster of blue aragonites in Malaval Cave © Gaëtan Rochez (UNamur)
Once again, a few fragments were collected, including a large bluish stalactite found broken on the cave floor. A series of microscopic observations and mineralogical and geochemical analyses were carried out. The first striking finding was that several blue fragments contained no minerals other than aragonite, suggesting that, unlike the green ones from Marcou, it was the aragonite itself that was coloured by the presence of metallic elements. After examining the analyses, three of these elements stood out: copper, commonly cited as the cause of blue colouring in aragonite, as well as zinc and lead.
While copper appears to be the main cause of the blue colouration, zinc and lead also play a role here.
Zinc is largely present in the form of deep blue amorphous phases, which are only found in some of the blue fragments studied. The presence of these phases, linked to the oxidation of nearby zinc-rich deposits, generates variations in the blue colour at the microscopic level, as revealed by optical microspectrophotometry.
Lead also has a marked colouring power, producing green to blue hues, but statistical analysis of coloured and uncoloured areas shows that these colours only appear in the absence of zinc, which seems to inhibit lead-induced colouring. This study clearly demonstrates that, even if a problem seems easy to explain at first glance, it can sometimes hide unexpected subtleties that need to be explored in greater depth in order to uncover all its secrets.
Gypsum from the Cigalère: the underground rainbow
The Cigalère Cave is one of a kind. Not only does it contain impressive quantities of gypsum, a calcium sulphate found in certain caves, but this gypsum also displays a wide variety of colours rarely seen in nature. Because of this rarity, the cave is particularly well protected, to the point that we were not allowed to collect any fragments from inside it.
This study was therefore the ideal opportunity to test the Geology Department's new acquisition: a portable X-ray fluorescence spectrometer (pXRF), which allows rapid, in situ, and above all completely non-destructive analysis of coloured speleothems.

Photos - pXRF analysis of a blue stalactite core (left) and a yellow flowstone (right) in the Cigalère Cave © Stéphane Pire (UNamur)
A total of five sites of interest were selected in the Cigalère for the diversity of colours found there. The pXRF revealed the presence of several metals.
At Cascade Noire, for example, a high concentration of iron in the form of oxides and sulphates was detected, which are responsible for the black and orange colouring of the gypsum, respectively.
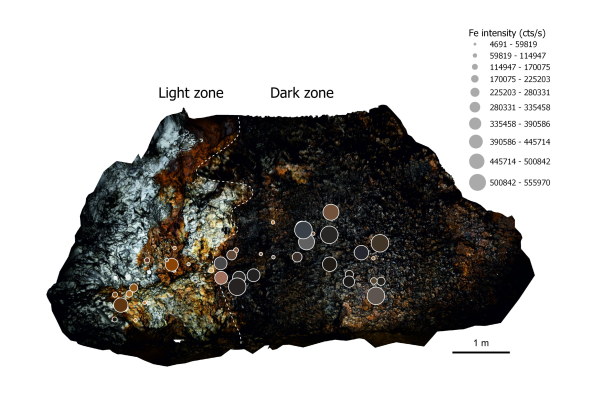
Black is also found in the Chapelle de Donnea, but contrary to what one might think, no iron has been detected. Here, it is manganese in the form of oxides that is responsible for the colouration. This observation is interesting because it clearly demonstrates that black colouration in gypsum, two phenomena that appear similar at first glance, can have very different causes, hence the importance of being able to carry out analyses directly in the field.
A little further downstream, blue dominates along the main gallery, and analyses have shown strong similarities with the blue speleothems of Malaval, with a marked influence of copper and potentially zinc.
All this highlights that, despite certain limitations of the device, this type of non-destructive analysis method is a very valuable tool for studying rare, fragile, precious, or protected objects, of which the Cigalère cave is an excellent example!
The research team
Martin Vlieghe's doctoral thesis on "The origin(s) of colored speleothems in caves," supervised by Professor Johan Yans and in collaboration with Gaëtan Rochez, began in February 2022. All three researchers are members of the Faculty of Sciences, Department of Geology at UNamur and the ILEE Research Institute.
ILEE (Institute of Life, Earth and Environment) is directly involved in issues related to the study and preservation of the environment, to which this subject is directly linked.
The various analyses were carried out with the support of UNamur's technological platforms:
- Physicochemical characterization (PC²)
- Lasers, optics, and spectroscopy (LOS)
- Electron microscopy
- Synthesis, Irradiation and Analysis of Materials (SIAM)
Some analyses were carried out in partnership with KUL, MRScNB and UMontpellier, and access to the caves was provided by the Association Mont Marcou, the Malaval Association and the Association de Recherche souterraine du Haut Lez.
This thesis was originally funded by the ILEE institute and institutional funds from UNamur, and by an Aspirant F.R.S. - FNRS grant (FC 50205) since October 2023.
It is also closely linked to the new research partnership supported by the RELIEF network (Réseau d’Échanges et de Liaisons entre Institutions d’Enseignement supérieur Francophones), the ILEE research institute at UNamur, and EDYTEM (Environnements, Dynamiques et Territoires de Montagne, Université Savoie Mont Blanc). Mobility programs between these entities will strengthen a common research area: the study of the critical zone, the most superficial zone of the Earth, where rocks, water, air, and living organisms interact. The perspective is to develop other transdisciplinary research areas and potential teaching projects in the field of environmental sciences and sustainable development.
Being curious about the Earth and the natural world: a key to meeting tomorrow's challenges!
Studying geology means developing a solid foundation in physics, chemistry, and biology in order to understand the Earth, from its internal dynamics to surface processes and their interactions with our environment and human activities.
Thanks to their interdisciplinary training, geologists are ideally positioned to perform a variety of roles that require a rigorous scientific approach to solving complex problems (research and development, project management, consulting, and education).
What are the advantages of studying at UNamur?
- Practical training and numerous field activities
- Strong scientific foundations
- Immersion in geology from block 1
- The possibility of ERASMUS from block 3 onwards
- Close contact with teachers
The advantages of studying in Namur
- A practical training and lots of field activities
- Strong scientific foundations
- Immersion in geology from the 1st year
- ERASMUS possible from the 3rd year onwards
- Close contact with teachers

50 years of electron microscopy at UNamur
50 years of electron microscopy at UNamur
In the hall of the Faculty of Medicine still sits the first transmission electron microscope, a Philips EM300 used at the Facultés Notre-Dame de la Paix in the 70s. The history of electron microscopy at UNamur began, but the real start was made in 1975 with the acquisition of three more microscopes: two transmission and one scanning. In February 2018, when the technology platforms were created, this department was attached to the MORPH-IM platform, and in April 2024 became the independent "Electron Microscopy" platform, UNamur's 11th technology platform
.
Electron microscopy has become an indispensable technique in many fields of research as varied as materials science (metallurgy, crystallography, etc.) or life sciences (cell biology, medicine, etc.). The principle is to use accelerated electrons, instead of a beam of light as in a conventional photonic microscope, to make much smaller structures observable, right up to atomic resolution.
This technique therefore makes it possible to obtain structural information through imaging, but not only. Thanks to the interaction of electrons with the atoms of the material, other emitted signals can also be analyzed to obtain additional information, for example, on the elemental composition (X-ray analyzer) or crystallography (detector for backscattered electron diffraction) of the sample.
Cutting-edge human resources and equipment at the service of research
Since 1975 and the initial three electron microscopes, two transmission - Philips EM301 and EM201- and one scanning - JEOL JSM-35 - equipped in 1980 with an X-ray analyzer, electron microscopy at UNamur has evolved in step with the microscopes acquired. A new scanning microscope - a Philips XL20 equipped with an X-ray analyzer - replaced the old one in 1991. Then, in 1999, a new transmission microscope was acquired - a Tecnai10 (FEI)- which was the subject of an article in the newspaper "Le Soir".
The article "Images of the infinitely small shown live" states: "It's not every day that the institution equips itself with a new transmission electron microscope, what's more, the first of its generation in Belgium. (...) The big step of this microscope of a new kind? Its image acquisition process. Whereas previously, images observed on film were fixed using a photographic process, it's now a digital camera coupled to a powerful computer that operates".
Professor Yves Poumay, interviewed at the time, explains, "Some researchers from other universities come to us, not because they don't have equivalent equipment at home, but because it's less accessible or less good"". At UNamur, they "not only provide researchers with a microscope, but also a team of laboratory technicians and an accompanying engineer, which constitutes a rather unusual human framework that is as valuable as the new microscope itself."
The platform's philosophy has not changed, with researchers from all walks of life, but also companies, still and always calling on its expertise.
The modernization of equipment continued in the following years with the acquisition of several scanning microscopes a JEOL JSM 7500F equipped with an X-ray analyzer in 2007 and a JEOL JSM 6010LV in 2012. The latter was very recently modified in 2023 with the acquisition of an X-ray analyzer (SDD QUANTAX, Bruker) and a detector for backscattered electron diffraction (eFlash QUANTAX, Bruker) as part of the inter-university research platform for the energy transition (RRF).
In 2016, a Tecnai20 (FEI), equipped with an X-ray analyzer (SDD QUANTAX, Bruker) mounted in 2021, complements the Tecnai10 for transmission microscopy analyses.
New technologies for tomorrow's analysis
As part of the BIOGREEN technology platform of excellence in 2024, the acquisition of a JEOL JEM F200 cryogenic transmission microscope will add value to the existing fleet of instruments. This new microscope enables the analysis of sensitive materials while minimizing electron beam damage. Thanks to specific preparation methods such as sample vitrification (Leica EM GP2 automatic immersion freezer) coupled with cryo-microscopy, it is thus possible to obtain information on the structure of biological objects (proteins, ribosomes, etc.).
For completeness, the development of electron microscopy has been accompanied by the acquisition of all the equipment required for sample preparation. Some equipment, such as the microtomization equipment, is original (1975), but is in the process of being replaced, while others are more recent, such as the sputtering device (QUORUM QT 150T/ES in 2015).
Microscopy members over the years
In 1975, Professor Robert Leloup created the Unité Interfacultaire de Microscopie Électronique, thanks to a substantial budget allocated by the institution. As today, it is located at the corner of rue Grafé and Place du Palais de Justice in the Faculty of Medicine. In 1998, Prof. Yves Poumay took over from Prof. Poumay until 2010, when Dr. Jean-François Colomer became head of the Microscopy Department, and with him a new team was put in place. In 2011, platform engineer Corry Charlier was hired. In 2017, Caroline De Bona joins as a technician. In 2025, the facility becomes part of the FWB's BIOGREEN technology platform of excellence, and a research logistician, Emir Topagolu, joins the team.

The team: Jean-François Colomer, Caroline De Bona, Corry Charlier and Emir Topaloglu
The "electron microscopy" platform boasts state-of-the-art equipment and remarkable expertise, which it has been sharing for 50 years with all UNamur members who wish to use these microscopic techniques for observation or analysis purposes, from both a research and teaching perspective, whether in materials science or life science. It also makes its expertise available to research organizations from other academic establishments and to companies or industries for the analysis of their samples.
A little technique
There are two main classes of electron microscope:
- The transmission electron microscope, where the electron beam passes through the sample. The sample must therefore be very thin. It is the transmitted and/or scattered electrons that are detected to form a cross-sectional image of it;
- the scanning electron microscope, whose electron beam scans the surface of the sample. The sample is therefore massive. It is the electrons ejected by the sample that are detected to form an image of its surface.
This article is taken from the "The Day When" section of Omalius magazine #35 (July 2025).


Producing "green" hydrogen from water from the Meuse River? It's now possible!
Producing "green" hydrogen from water from the Meuse River? It's now possible!
At UNamur, research is not confined to laboratories. From physics to political science, robotics, biodiversity, law, AI, and health, researchers collaborate daily with numerous stakeholders in society. The goal? Transform ideas into concrete solutions to address current challenges.

Focus #2 | What if our rivers became a source of clean energy for the future?
An international team of chemistry researchers, led by Dr. Laroussi Chaabane and Prof. Bao-Lian Su, has just demonstrated that it is possible to produce "green" hydrogen using natural water and sunlight. These findings have been published in the prestigious Chemical Engineering Journal.
When sunlight becomes a source of clean energy
Faced with climate change, pollution, and energy shortages, the search for alternatives to fossil fuels has become a global priority in order to achieve carbon neutrality by 2050. Among the solutions being considered, green hydrogen appears to be a particularly promising energy carrier: it has a high energy density and can be produced without greenhouse gas emissions. Today, most of the world's hydrogen (around 87 million tons produced in 2020) is obtained through costly and polluting electrochemical processes, mainly used by the chemical industry or fuel cells. Hence the major interest in more sustainable methods.
Water photocatalysis: the "Holy Grail" of chemistry
Producing hydrogen and oxygen directly from water using light, a process known as photocatalysis of water, is often referred to as the "Holy Grail of chemistry" because it is so complex to master. At the University of Namur, researchers at the Laboratory of Inorganic Materials Chemistry (CMI), part of the Nanomaterials Chemistry Unit (UCNANO) and the Namur Institute of Structured Matter (NISM), have taken a decisive step forward. They have demonstrated that it is possible to use natural water, and no longer just ultrapure water, to produce green hydrogen under the action of sunlight.

The core of the process is based on an innovative photocatalyst, which acts as a kind of "chemical pair of scissors" capable of splitting water molecules into hydrogen and oxygen—an area in which the CMI laboratory has recognized expertise.
A 3D photocatalyst based on graphene and gold
The new material developed is a three-dimensional (3D) photocatalyst based on titanium oxide, graphene, and gold nanoparticles. This 3D architecture allows for better light absorption and more efficient generation of free electrons, which are essential for triggering the water dissociation reaction. One of the main challenges lies in the use of natural water, which contains minerals, salts, and organic compounds that can disrupt the process. To address this challenge, the researchers tested their device with water from several Belgian rivers: the Meuse, the Sambre, the Scheldt, and the Yser.

A remarkable result and a first in Belgium!
The performance achieved is almost equivalent to that measured with pure water.
This is a first in Belgium, opening up concrete prospects for the sustainable use of local natural resources!
The full article, "Synergistic four physical phenomena in a 3D photocatalyst for unprecedented overall water splitting," is available in open access.
International recognition
This scientific breakthrough also earned Dr. Laroussi Chaabane the award for best poster at the 4th International Colloids Conference (San Sebastián, Spain, July 2025), highlighting the impact and originality of this work.
An international research team
- University of Namur, Faculty of Sciences, UCNANO, Laboratory of Inorganic Materials Chemistry (CMI) and Namur Institute of Structured Matter (NISM), Belgium | Principal Investigator (PI) | Professor Bao Lian SU; Postdoctoral Researcher | Dr. Laroussi Chaabane
- Institute of Organic Chemistry, Phytochemistry Center, Academy of Sciences, Bulgaria
- Department of Organic Chemistry (MSc), Loyola Academy, India
- Free University of Brussels (ULB) and Flanders Make, Department of Applied Physics and Photonics, Brussels Photonics, Belgium
- University of Quebec in Montreal (UQAM), Department of Chemistry, Montreal, Quebec, Canada
- National Institute for Scientific Research - Energy Materials Telecommunications Center (INRS-EMT), Varennes, Quebec, Canada
- Wuhan University of Technology, National Laboratory for Advanced Technologies in Materials Synthesis and Processing, China
What next?
At this stage, the study constitutes proof of concept demonstrating the feasibility of the process. It illustrates the excellence of chemical engineering and nanomaterials research at UNamur, as well as its potential for sustainable energy applications. A new study is underway to evaluate the performance of the process with seawater, a key step towards large-scale green hydrogen production.
State-of-the-art equipment
The analyses carried out were made possible thanks to the equipment available at UNamur's Physico-Chemical Characterization (PC²), Electron Microscopy, and Material Synthesis, Irradiation, and Analysis (SIAM) technology platforms. UNamur's technology platforms house state-of-the-art equipment and are accessible to the scientific community as well as to industries and companies.
The authors would like to thank the Wallonia Public Service (SPW) for its ongoing commitment to scientific research and innovation in Wallonia, enabling UNamur to develop technological solutions with a significant societal and environmental impact.
From fundamental to applied research, UNamur demonstrates every day that research is a driver of transformation. Thanks to the commitment of its researchers, the support of its partners from all walks of life, funders, industrial partners, and a solid ecosystem of valorization, UNamur actively participates in shaping a society that is open to the world, more innovative, more responsible, and more sustainable.
To go further
This article complements our publication "Research and innovation: major assets for the industrial sector" taken from the Issues section of Omalius magazine #39 (December 2025).

Colourful speleothems: treasures hidden deep within the earth
Colourful speleothems: treasures hidden deep within the earth
Well hidden from passersby, caves nevertheless conceal particularly aesthetic secrets. For the past four years, Martin Vlieghe has been pursuing a PhD in geology at UNamur. He is exploring the origin of the surprisingly varied colours of certain concretions nestled in the heart of Belgian and French caves. Together with Prof. Johan Yans and Gaëtan Rochez, he samples, observes, and analyses these magnificent objects with the aim of uncovering the mysteries they conceal.

Photo: Green speleothems in the Aven du Mont Marcou (Hérault, France) © Stéphane Pire, Gaëtan Rochez (UNamur)
Speleothems, for instance stalactites and stalagmites, are commonly composed of calcite or aragonite (CaCO3). This mineral compound comes directly from the rock in which the cave was formed and naturally has a white to brownish colour. However, speleothems can sometimes exhibit unique and unusual colours. From yellow to black, blue, red, green, and even purple, there is something for everyone!
Such a diversity of colours reflects the many possible causes: mineralogical, chemical, biological, or even physical. A speleothem, like any natural formation, is never perfectly pure. Their deposition process, through the precipitation of calcium carbonate dissolved in water, is necessarily accompanied by the deposition of numerous impurities carried along with the water circulating underground. Even if these impurities are sometimes too low in concentration or simply uncoloured, they can still have a visible impact on the colour.
OK, but what is the point?
The formation of speleothems is very often linked to impurities dissolved in groundwater. Therefore, studying coloured speleothems provides valuable information about potential contamination of surface water with heavy metals or other harmful organic compounds, which in some cases may be consumed by residents. It is therefore a simple and direct way to identify areas with potentially contaminated water and to determine whether this contamination poses an environmental or health risk.
This is the objective of Martin Vlieghe's thesis: to apply a range of cutting-edge analytical techniques to samples of these speleothems to determine these causes and propose an explanation for the origin of the colouring elements.
Here are a few examples.
Green from the Aven du Marcou: the influence of nickel
An initial project explored the green speleothems of the Aven du Marcou (see photo above). Located in the Hérault department of France, this chasm is well known in the area for its series of impressive shafts, the largest of which is over 100 meters deep. It also has a tiny chamber hidden at the top of a steep wall, which houses an impressive concentration of deep green speleothems. After all the effort of descending and climbing ropes to progress through this very vertical cave, what a wonderful reward to discover this true underground gem! Once the initial wonder has passed, it's time to get to work! We observe, describe, interpret, and collect a few green fragments from the ground, while respecting the integrity of the site as much as possible. Back in Belgium, it's time to move on to the analyses.
Careful observation of the recovered fragments quickly reveals the presence of green minerals in the outer part of the speleothems, which are easily associated with the green colour observed. These minerals, which are deposited in platelets parallel to the white aragonite (CaCO₃), turn out to be nepouite crystals, a nickel phyllosilicate ((Ni,Mg)₃Si₂O₅(OH)₄) usually found in marine volcanic rocks.

The discovery is all the more surprising given that there are no nickel deposits in the vicinity of the cave! Further study of the composition of the nepouite reveals that they contain a high concentration of zinc, which is also very unusual and suggests that they are in fact quite different from those commonly mined in volcanic deposits. Finally, this mystery was solved by a thorough examination of the rock outcrops in the immediate vicinity of the cave. Just above the cave are siliceous deposits particularly rich in pyrite, an iron sulphide commonly found in this type of settingst. Analysis of these sulphides reveals high concentrations of nickel, which is also found in the natural water source closest to the cave.
The result of this "investigation" and final explanation: nepouite was able to settle underground through the dissolution of various chemical elements contained in the pyrite of the overlying rocks, which were transported into the cave by surface water and were able to crystallize on site.
Malaval blues: when metals interact
The Malaval cave is very different from the Aven du Marcou. Located in Lozère (France), it extends largely along a high underground river that winds beneath the Cévennes massif. At the bend of a meander, one can find magnificent blue speleothems.
As in the Aven du Marcou, the coloured speleothems are found only in two specific locations in the cave and nowhere else, suggesting that the origin of the chromophore elements is probably very localized.

Photos - Left: Blue stalagmite in Malaval Cave. Right: Cluster of blue aragonites in Malaval Cave © Gaëtan Rochez (UNamur)
Once again, a few fragments were collected, including a large bluish stalactite found broken on the cave floor. A series of microscopic observations and mineralogical and geochemical analyses were carried out. The first striking finding was that several blue fragments contained no minerals other than aragonite, suggesting that, unlike the green ones from Marcou, it was the aragonite itself that was coloured by the presence of metallic elements. After examining the analyses, three of these elements stood out: copper, commonly cited as the cause of blue colouring in aragonite, as well as zinc and lead.
While copper appears to be the main cause of the blue colouration, zinc and lead also play a role here.
Zinc is largely present in the form of deep blue amorphous phases, which are only found in some of the blue fragments studied. The presence of these phases, linked to the oxidation of nearby zinc-rich deposits, generates variations in the blue colour at the microscopic level, as revealed by optical microspectrophotometry.

Lead also has a marked colouring power, producing green to blue hues, but statistical analysis of coloured and uncoloured areas shows that these colours only appear in the absence of zinc, which seems to inhibit lead-induced colouring. This study clearly demonstrates that, even if a problem seems easy to explain at first glance, it can sometimes hide unexpected subtleties that need to be explored in greater depth in order to uncover all its secrets.
Gypsum from the Cigalère: the underground rainbow
The Cigalère Cave is one of a kind. Not only does it contain impressive quantities of gypsum, a calcium sulphate found in certain caves, but this gypsum also displays a wide variety of colours rarely seen in nature. Because of this rarity, the cave is particularly well protected, to the point that we were not allowed to collect any fragments from inside it.
This study was therefore the ideal opportunity to test the Geology Department's new acquisition: a portable X-ray fluorescence spectrometer (pXRF), which allows rapid, in situ, and above all completely non-destructive analysis of coloured speleothems.

Photos - pXRF analysis of a blue stalactite core (left) and a yellow flowstone (right) in the Cigalère Cave © Stéphane Pire (UNamur)
A total of five sites of interest were selected in the Cigalère for the diversity of colours found there. The pXRF revealed the presence of several metals.
At Cascade Noire, for example, a high concentration of iron in the form of oxides and sulphates was detected, which are responsible for the black and orange colouring of the gypsum, respectively.

Black is also found in the Chapelle de Donnea, but contrary to what one might think, no iron has been detected. Here, it is manganese in the form of oxides that is responsible for the colouration. This observation is interesting because it clearly demonstrates that black colouration in gypsum, two phenomena that appear similar at first glance, can have very different causes, hence the importance of being able to carry out analyses directly in the field.
A little further downstream, blue dominates along the main gallery, and analyses have shown strong similarities with the blue speleothems of Malaval, with a marked influence of copper and potentially zinc.
All this highlights that, despite certain limitations of the device, this type of non-destructive analysis method is a very valuable tool for studying rare, fragile, precious, or protected objects, of which the Cigalère cave is an excellent example!
The research team
Martin Vlieghe's doctoral thesis on "The origin(s) of colored speleothems in caves," supervised by Professor Johan Yans and in collaboration with Gaëtan Rochez, began in February 2022. All three researchers are members of the Faculty of Sciences, Department of Geology at UNamur and the ILEE Research Institute.
ILEE (Institute of Life, Earth and Environment) is directly involved in issues related to the study and preservation of the environment, to which this subject is directly linked.
The various analyses were carried out with the support of UNamur's technological platforms:
- Physicochemical characterization (PC²)
- Lasers, optics, and spectroscopy (LOS)
- Electron microscopy
- Synthesis, Irradiation and Analysis of Materials (SIAM)
Some analyses were carried out in partnership with KUL, MRScNB and UMontpellier, and access to the caves was provided by the Association Mont Marcou, the Malaval Association and the Association de Recherche souterraine du Haut Lez.
This thesis was originally funded by the ILEE institute and institutional funds from UNamur, and by an Aspirant F.R.S. - FNRS grant (FC 50205) since October 2023.
It is also closely linked to the new research partnership supported by the RELIEF network (Réseau d’Échanges et de Liaisons entre Institutions d’Enseignement supérieur Francophones), the ILEE research institute at UNamur, and EDYTEM (Environnements, Dynamiques et Territoires de Montagne, Université Savoie Mont Blanc). Mobility programs between these entities will strengthen a common research area: the study of the critical zone, the most superficial zone of the Earth, where rocks, water, air, and living organisms interact. The perspective is to develop other transdisciplinary research areas and potential teaching projects in the field of environmental sciences and sustainable development.
Being curious about the Earth and the natural world: a key to meeting tomorrow's challenges!
Studying geology means developing a solid foundation in physics, chemistry, and biology in order to understand the Earth, from its internal dynamics to surface processes and their interactions with our environment and human activities.
Thanks to their interdisciplinary training, geologists are ideally positioned to perform a variety of roles that require a rigorous scientific approach to solving complex problems (research and development, project management, consulting, and education).
What are the advantages of studying at UNamur?
- Practical training and numerous field activities
- Strong scientific foundations
- Immersion in geology from block 1
- The possibility of ERASMUS from block 3 onwards
- Close contact with teachers
The advantages of studying in Namur
- A practical training and lots of field activities
- Strong scientific foundations
- Immersion in geology from the 1st year
- ERASMUS possible from the 3rd year onwards
- Close contact with teachers

50 years of electron microscopy at UNamur
50 years of electron microscopy at UNamur
In the hall of the Faculty of Medicine still sits the first transmission electron microscope, a Philips EM300 used at the Facultés Notre-Dame de la Paix in the 70s. The history of electron microscopy at UNamur began, but the real start was made in 1975 with the acquisition of three more microscopes: two transmission and one scanning. In February 2018, when the technology platforms were created, this department was attached to the MORPH-IM platform, and in April 2024 became the independent "Electron Microscopy" platform, UNamur's 11th technology platform
.
Electron microscopy has become an indispensable technique in many fields of research as varied as materials science (metallurgy, crystallography, etc.) or life sciences (cell biology, medicine, etc.). The principle is to use accelerated electrons, instead of a beam of light as in a conventional photonic microscope, to make much smaller structures observable, right up to atomic resolution.
This technique therefore makes it possible to obtain structural information through imaging, but not only. Thanks to the interaction of electrons with the atoms of the material, other emitted signals can also be analyzed to obtain additional information, for example, on the elemental composition (X-ray analyzer) or crystallography (detector for backscattered electron diffraction) of the sample.
Cutting-edge human resources and equipment at the service of research
Since 1975 and the initial three electron microscopes, two transmission - Philips EM301 and EM201- and one scanning - JEOL JSM-35 - equipped in 1980 with an X-ray analyzer, electron microscopy at UNamur has evolved in step with the microscopes acquired. A new scanning microscope - a Philips XL20 equipped with an X-ray analyzer - replaced the old one in 1991. Then, in 1999, a new transmission microscope was acquired - a Tecnai10 (FEI)- which was the subject of an article in the newspaper "Le Soir".
The article "Images of the infinitely small shown live" states: "It's not every day that the institution equips itself with a new transmission electron microscope, what's more, the first of its generation in Belgium. (...) The big step of this microscope of a new kind? Its image acquisition process. Whereas previously, images observed on film were fixed using a photographic process, it's now a digital camera coupled to a powerful computer that operates".
Professor Yves Poumay, interviewed at the time, explains, "Some researchers from other universities come to us, not because they don't have equivalent equipment at home, but because it's less accessible or less good"". At UNamur, they "not only provide researchers with a microscope, but also a team of laboratory technicians and an accompanying engineer, which constitutes a rather unusual human framework that is as valuable as the new microscope itself."
The platform's philosophy has not changed, with researchers from all walks of life, but also companies, still and always calling on its expertise.
The modernization of equipment continued in the following years with the acquisition of several scanning microscopes a JEOL JSM 7500F equipped with an X-ray analyzer in 2007 and a JEOL JSM 6010LV in 2012. The latter was very recently modified in 2023 with the acquisition of an X-ray analyzer (SDD QUANTAX, Bruker) and a detector for backscattered electron diffraction (eFlash QUANTAX, Bruker) as part of the inter-university research platform for the energy transition (RRF).
In 2016, a Tecnai20 (FEI), equipped with an X-ray analyzer (SDD QUANTAX, Bruker) mounted in 2021, complements the Tecnai10 for transmission microscopy analyses.
New technologies for tomorrow's analysis
As part of the BIOGREEN technology platform of excellence in 2024, the acquisition of a JEOL JEM F200 cryogenic transmission microscope will add value to the existing fleet of instruments. This new microscope enables the analysis of sensitive materials while minimizing electron beam damage. Thanks to specific preparation methods such as sample vitrification (Leica EM GP2 automatic immersion freezer) coupled with cryo-microscopy, it is thus possible to obtain information on the structure of biological objects (proteins, ribosomes, etc.).
For completeness, the development of electron microscopy has been accompanied by the acquisition of all the equipment required for sample preparation. Some equipment, such as the microtomization equipment, is original (1975), but is in the process of being replaced, while others are more recent, such as the sputtering device (QUORUM QT 150T/ES in 2015).
Microscopy members over the years
In 1975, Professor Robert Leloup created the Unité Interfacultaire de Microscopie Électronique, thanks to a substantial budget allocated by the institution. As today, it is located at the corner of rue Grafé and Place du Palais de Justice in the Faculty of Medicine. In 1998, Prof. Yves Poumay took over from Prof. Poumay until 2010, when Dr. Jean-François Colomer became head of the Microscopy Department, and with him a new team was put in place. In 2011, platform engineer Corry Charlier was hired. In 2017, Caroline De Bona joins as a technician. In 2025, the facility becomes part of the FWB's BIOGREEN technology platform of excellence, and a research logistician, Emir Topagolu, joins the team.

The team: Jean-François Colomer, Caroline De Bona, Corry Charlier and Emir Topaloglu
The "electron microscopy" platform boasts state-of-the-art equipment and remarkable expertise, which it has been sharing for 50 years with all UNamur members who wish to use these microscopic techniques for observation or analysis purposes, from both a research and teaching perspective, whether in materials science or life science. It also makes its expertise available to research organizations from other academic establishments and to companies or industries for the analysis of their samples.
A little technique
There are two main classes of electron microscope:
- The transmission electron microscope, where the electron beam passes through the sample. The sample must therefore be very thin. It is the transmitted and/or scattered electrons that are detected to form a cross-sectional image of it;
- the scanning electron microscope, whose electron beam scans the surface of the sample. The sample is therefore massive. It is the electrons ejected by the sample that are detected to form an image of its surface.
This article is taken from the "The Day When" section of Omalius magazine #35 (July 2025).

Agenda
Exploring SEM & TEM: From Fundamentals to Applications
The University of Namur - Electron Microscopy Platform - and JEOL are organizing a day dedicated to knowledge sharing and practical exploration of electron microscopy techniques, including scanning and transmission microscopy, cross-section preparation (CP), and FIB.

This event will provide students, researchers, and industry representatives with an opportunity to explore the evolution of electron microscopy, deepen their understanding of advanced analysis techniques, and participate in live demonstrations in a real research environment.
The program includes expert lectures on electron microscopy and analysis methods (EDS, EBSD, detectors), live SEM demonstrations, and a guided tour of the laboratory.
Participants will have the opportunity to interact with JEOL specialists and the electron microscopy platform during hands-on sessions and networking opportunities.
The event offers 1 ECTS credit to doctoral researchers and a certificate of participation upon request.


